indeterminate error-indeterminate error example?
indeterminate-error-indeterminate-error-examples,what is indeterminate-error,indeterminate-error example,characteristics of indeterminate-error, types of indeterminate-error,indeterminate-error,indeterminate-error
There square measure four freelance sources of indeterminate error within the within the peaks by height and width: putting the bottom line, activity the peak, activity the intermediate height, and activity the dimension. Perimeter ways, i.e., measuring instrument and cutting and consideration, have similar errors arising from putting the bottom line, tracing the height define, getting a reading, and, for cutting and consideration, variability of the paper thickness. In general, these errors rely upon peak form and peak space. The relative error in space decreases with increasing peak space. In most cases there's An optimum peak form which provides minimum error. though several factors have an effect on the optimum form, it ofttimes is within the vary of two to ten for the magnitude relation of height to dimension at half-height.
What do you know about error?
“Error” in Chemistry is outlined because the distinction between actuality result (or accepted true result) and also the measured result. If the error within the analysis is massive, serious consequences might result. As dependability, dependable, and accuracy square measure the premise of analytical chemistry.
Classification of Errors:
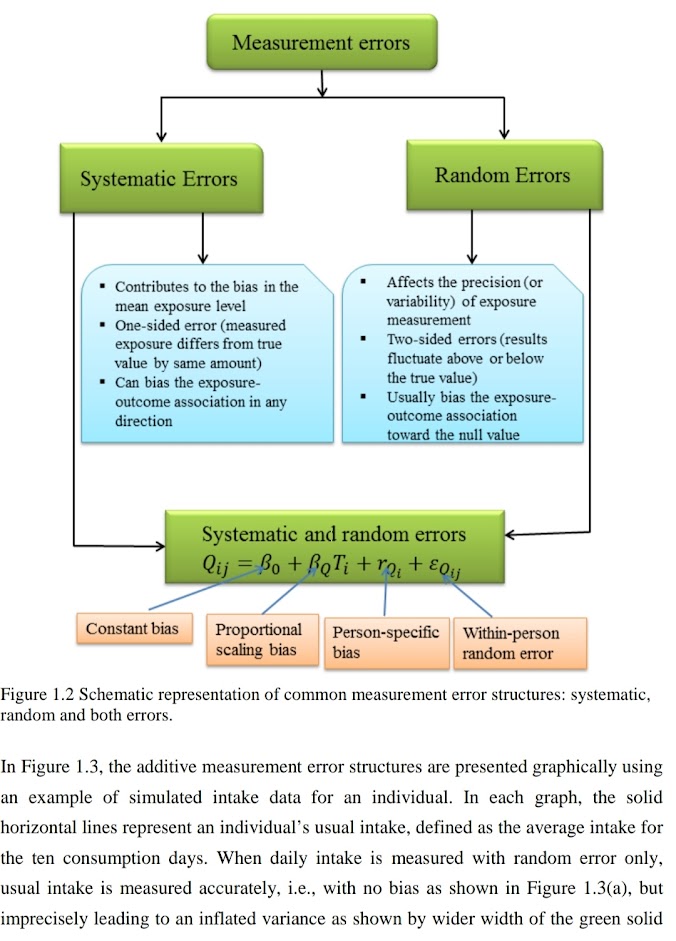
Errors square measure classified in 2 varieties – Systemic (Determinate) and Random (Indeterminate) errors
🔵Systemic (Determinate) errors:
Errors which might be avoided or whose magnitude will be determined is termed as general errors. It will be determinable and presumptively will be either avoided or corrected. general errors more classified as
🔵Operational and private error
🔵Instrumental error
🔵Errors of methodology
🔵Additive or proportional error
🔵Operational and private error:
Errors that the individual analyst is accountable and don't seem to be connected with the tactic or procedure is termed as personal errors e.g. unable to guage colour modification
When errors occur throughout operation is termed as operational error e.g. transfers of answer, effervescence, incomplete drying, under weighting of precipitates, over weighing of precipitates, and short cooling of precipitates. These errors square measure physical in nature and occur once sound analytical techniques isn't followed
🔵Instrumental and chemical agent errors:
Errors occur because of faulty instrument or chemical agent containing impurities e.g. un-calibrated weights, un-calibrated measuring instrument, pipet and activity flasks.
🔵Errors of Method:
When errors occur because of methodology, it's tough to correct. In quantitative analysis, error happens because of quality of precipitates, co-precipitates, post-precipitates, decomposition, and volatilisation.
In metric analysis errors occur because of failure of reaction, facet reaction, reaction of substance apart from the constituent being determined, distinction between ascertained finish purpose and also the ratio equivalence purpose of a reaction.
🔵Additive or proportional errors:
Additive error doesn't rely upon constituent gift within the determination e.g. loss in weight of a vessel within which a precipitate is lit.
Proportional error depends on the number of the constituent e.g. impurities in commonplace compound.
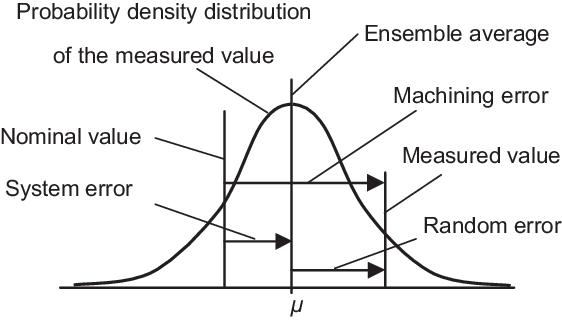
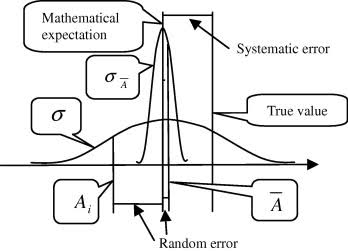
🔵Random Errors:(indeterminate)
It happens accidentally or indiscriminately therefore referred to as as indeterminate or accidental or random error. Analyst has no management during this error. It follows a random distribution and a mathematical law of likelihood will be applied.






EmoticonEmoticon